|
Quantum mechanics is essential to understand the behavior of systems at atomic length scales and smaller. For example,
if classical
mechanics governed the workings of an atom, electrons would rapidly travel towards and collide
with the nucleus,
making stable atoms impossible. However, in the natural world the electrons
normally remain in an unknown orbital path around the nucleus, defying classical
electromagnetism.
Quantum mechanics was initially developed to provide a better explanation of
the atom, especially the spectra of
light emitted by different atomic species. The quantum theory of the
atom was developed as an explanation for the electron's staying in its orbital, which could not
be explained by Newton's laws of motion and by Maxwell's laws of classical
electromagnetism.
In the formalism of quantum mechanics, the state of a system at a given time
is described by a complex wave function (sometimes referred to as orbitals
in the case of atomic electrons), and more generally, elements of a complex vector space. This abstract
mathematical object allows for the calculation of probabilities of outcomes of concrete experiments.
For example, it allows one to compute the probability of finding an electron in
a particular region around the nucleus at a particular time. Contrary to
classical mechanics, one can never make simultaneous predictions of conjugate
variables, such as position and momentum, with arbitrary accuracy. For
instance, electrons may be considered to be located somewhere within a region of
space, but with their exact positions being unknown. Contours of constant
probability, often referred to as “clouds” may be drawn around the nucleus of an
atom to conceptualize where the electron might be located with the most
probability. Heisenberg's uncertainty principle quantifies the
inability to precisely locate the particle.
The other exemplar that led to
quantum mechanics was the study of electromagnetic
waves such as light. When it was found in 1900 by Max Planck that the energy
of waves could be described as consisting of small packets or quanta, Albert Einstein
exploited this idea to show that an electromagnetic wave such as light could be
described by a particle called the photon with a discrete energy dependent on its
frequency. This led to a theory of unity between subatomic particles
and electromagnetic waves called wave–particle duality in which
particles and waves were neither one nor the other, but had certain properties
of both. While quantum mechanics describes the world of the very small, it also
is needed to explain certain “macroscopic quantum systems” such as superconductors and
superfluids.
Broadly speaking, quantum mechanics incorporates four classes of phenomena
that classical physics cannot account for: (I) the quantization (discretization) of certain physical quantities, (II)
wave-particle duality, (III) the uncertainty
principle, and (IV) quantum entanglement. Each of these
phenomena is described in detail in subsequent sections.
The history of quantum mechanics[2] began
essentially with the 1838 discovery of cathode rays by Michael Faraday, the 1859 statement of the black body radiation problem by Gustav Kirchhoff, the
1877 suggestion by Ludwig Boltzmann that the energy states of a
physical system could be discrete, and the 1900 quantum hypothesis by Max Planck that any energy is
radiated and absorbed in quantities divisible by discrete ‘energy elements’, E,
such that each of these energy elements is proportional to the frequency ν with which they
each individually radiate energy, as
defined by the following formula
where h is Planck's Action Constant. Although
Planck insisted[3] that this was simply
an aspect of the processes of absorption and emission of radiation and had
nothing to do with the physical reality of the radiation itself, in 1905, to
explain the photoelectric effect (1839), i.e. that
shining light on certain materials can function to eject electrons from the
material, Albert
Einstein[4] postulated, as based
on Planck’s quantum hypothesis, that light
itself consists of individual quanta, which later came to be called photons (1926). From
Einstein's simple postulation was borne a flurry of debating, theorizing and
testing, and thus, the entire field of quantum physics.
The modern world of physics is founded on the to tested and demonstrably
sound theories of general relativity and quantum mechanics —theories which
appear to contradict one another. The defining postulates of both Einstein's
theory of relativity and quantum theory are indisputably supported by rigorous
and repeated empirical evidence. However, while they do not directly contradict
each other theoretically (at least with regard to primary claims), they are
resistant to being incorporated within one cohesive model.
Einstein himself is well known for rejecting some of the claims of quantum
mechanics. While clearly inventive in this field, he did not accept the more
philosophical consequences and interpretations of quantum mechanics, such as the
lack of deterministic causality
and the assertion that a single subatomic particle can occupy numerous areas of
space at one time. He also was the first to notice some of the apparently exotic
consequences of entanglement and used them to formulate
the Einstein-Podolsky-Rosen
paradox, in the hope of showing that quantum mechanics had unacceptable
implications. This was 1935, but in 1964 it was shown by John Bell (see Bell
inequality) that Einstein's assumption was correct, but had to be completed
by hidden variables and thus based on wrong philosophical assumptions.
According to the paper of J. Bell and the Copenhagen interpretation (the common
interpretation of quantum mechanics by physicists for decades), and contrary to
Einstein's ideas, quantum mechanics was
The Einstein-Podolsky-Rosen paradox shows in any case that there exist
experiments by which one can measure the state of one particle and
instantaneously change the state of its entangled partner, although the two
particles can be an arbitrary distance apart; however, this effect does not
violate causality, since no
transfer of information happens. These experiments are the basis of some of the
most topical applications of the theory, quantum cryptography, which works well,
although at small distances of typically  1000 km, being on the market since 2004.
1000 km, being on the market since 2004.
There do exist quantum theories which incorporate special
relativity—for example, quantum electrodynamics (QED), which is
currently the most accurately tested physical theory [5]—and these lie at the
very heart of modern particle physics. Gravity is negligible in
many areas of particle physics, so that unification between general relativity
and quantum mechanics is not an urgent issue in those applications. However, the
lack of a correct theory of quantum gravity is an important issue in cosmology.
Inconsistencies arise when one tries to join the quantum laws with general
relativity, a more elaborate description of spacetime which incorporates gravitation. Resolving these inconsistencies has
been a major goal of twentieth- and twenty-first-century physics. Many prominent
physicists, including Stephen Hawking, have labored in the attempt to
discover a "Grand Unification Theory" that
combines not only different models of subatomic physics, but also derives the
universe's four forces—the strong force, electromagnetism, weak force, and gravity— from a single force or
phenomenon.
Predictions of quantum mechanics have been verified experimentally to a very
high degree of accuracy. Thus, the current logic of correspondence principle between
classical and quantum mechanics is that all objects obey laws of quantum
mechanics, and classical mechanics is just a quantum mechanics of large systems
(or a statistical quantum mechanics of a large collection of particles). Laws of
classical mechanics thus follow from laws of quantum mechanics at the limit of
large systems or large quantum numbers.
Main differences between classical and quantum theories have already been
mentioned above in the remarks on the Einstein-Podolsky-Rosen paradox. Essentially the
difference boils down to the statement that quantum mechanics is coherent (addition of amplitudes), whereas classical
theories are incoherent (addition of intensities). Thus,
such quantities as coherence lengths and coherence times come into
play. For microscopic bodies the extension of the system is certainly much
smaller than the coherence length; for macroscopic bodies one expects that it
should be the other way round.
This is in accordance with the following observations:
Many “macroscopic” properties of “classic” systems are direct consequences of
quantum behavior of its parts. For example, stability of bulk matter (which
consists of atoms and molecules
which would quickly collapse under electric forces alone), rigidity of this
matter, mechanical, thermal, chemical, optical and magnetic properties of this
matter—they are all results of interaction of electric charges under the rules of quantum
mechanics.
While the seemingly exotic behavior of matter posited by quantum mechanics
and relativity theory become more apparent when dealing with extremely
fast-moving or extremely tiny particles, the laws of classical “Newtonian”
physics still remain accurate in predicting the behavior of surrounding
(“large”) objects—of the order of the size of large molecules and bigger—at
velocities much smaller than the velocity of light.
There are numerous mathematically equivalent formulations of quantum
mechanics. One of the oldest and most commonly used formulations is the transformation theory
proposed by Cambridge theoretical physicist Paul Dirac, which unifies and generalizes the two
earliest formulations of quantum mechanics, matrix mechanics (invented by Werner
Heisenberg)[6] and wave
mechanics (invented by Erwin Schrödinger).
In this formulation, the instantaneous state of a quantum system encodes
the probabilities of its measurable properties, or "observables". Examples of observables include energy, position, momentum, and angular momentum. Observables can be either continuous
(e.g., the position of a particle) or discrete (e.g., the energy of an electron
bound to a hydrogen atom).
Generally, quantum mechanics does not assign definite values to observables.
Instead, it makes predictions about probability distributions; that is,
the probability of obtaining each of the possible outcomes from measuring an
observable. Oftentimes these results are skewed by many causes, such as dense probability
clouds or quantum state nuclear attraction. Much of the time, these small
anomalies are attributed to different causes such as quantum
dislocation. Naturally, these probabilities will depend on the quantum state
at the instant of the measurement. When the probability amplitudes of four or
more quantum nodes are similar, it is called a quantum
parallelism. There are, however, certain states that are associated with a
definite value of a particular observable. These are known as "eigenstates" of
the observable ("eigen" can be roughly translated from German as inherent or
as a characteristic). In the everyday world, it is natural and intuitive to
think of everything being in an eigenstate of every observable. Everything
appears to have a definite position, a definite momentum, and a definite time of
occurrence. However, quantum mechanics does not pinpoint the exact values for
the position or momentum of a certain particle in a given space in a finite
time; rather, it only provides a range of probabilities of where that particle
might be. Therefore, it became necessary to use different words for (a) the
state of something having an uncertainty relation and (b) a state that
has a definite value. The latter is called the "eigenstate" of the
property being measured.
For example, consider a free particle. In quantum mechanics, there is wave-particle duality so the properties
of the particle can be described as a wave. Therefore, its quantum state can be
represented as a wave, of arbitrary shape
and extending over all of space, called a wave function. The position and momentum of the
particle are observables. The Uncertainty Principle of quantum
mechanics states that both the position and the momentum cannot simultaneously
be known with infinite precision at the same time. However, one can measure just
the position alone of a moving free particle creating an eigenstate of position
with a wavefunction that is very large at a particular position
x, and almost zero everywhere else. If one performs a position
measurement on such a wavefunction, the result x will be obtained with
almost 100% probability. In other words, the position of the free particle will
almost be known. This is called an eigenstate of position (mathematically more
precise: a generalized eigenstate (eigendistribution) ). If the
particle is in an eigenstate of position then its momentum is completely
unknown. An eigenstate of momentum, on the other hand, has the form of a plane wave. It can be shown that
the wavelength is equal to
h/p, where h is Planck's constant and p is the
momentum of the eigenstate. If the particle is in an eigenstate of
momentum then its position is completely blurred out.
Usually, a system will not be in an eigenstate of whatever observable we are
interested in. However, if one measures the observable, the wavefunction will
instantaneously be an eigenstate (or generalized eigenstate) of that observable.
This process is known as wavefunction collapse. It involves
expanding the system under study to include the measurement device, so that a
detailed quantum calculation would no longer be feasible and a classical
description must be used. If one knows the corresponding wave function at the
instant before the measurement, one will be able to compute the probability of
collapsing into each of the possible eigenstates. For example, the free particle
in the previous example will usually have a wavefunction that is a wave packet centered around
some mean position x0, neither an eigenstate of position nor
of momentum. When one measures the position of the particle, it is impossible to
predict with certainty the result that we will obtain. It is probable, but not
certain, that it will be near x0, where the amplitude of the
wave function is large. After the measurement is performed, having obtained some
result x, the wave function collapses into a position eigenstate centered
at x.
Wave functions can change as time progresses. An equation known as the Schrödinger
equation describes how wave functions change in time, a role similar to Newton's second law in classical
mechanics. The Schrödinger equation, applied to the aforementioned example of
the free particle, predicts that the center of a wave packet will move through
space at a constant velocity, like a classical particle with no forces acting on
it. However, the wave packet will also spread out as time progresses, which
means that the position becomes more uncertain. This also has the effect of
turning position eigenstates (which can be thought of as infinitely sharp wave
packets) into broadened wave packets that are no longer position
eigenstates.
Some wave functions produce probability distributions that are constant in
time. Many systems that are treated dynamically in classical mechanics are
described by such "static" wave functions. For example, a single electron in an unexcited atom is pictured classically as a particle moving in a
circular trajectory around the atomic nucleus, whereas in quantum mechanics it
is described by a static, spherically symmetric wavefunction
surrounding the nucleus (Fig. 1). (Note that only the lowest
angular momentum states, labeled s, are spherically symmetric).
The time evolution of wave functions is deterministic in the sense that, given a
wavefunction at an initial time, it makes a definite prediction of what the
wavefunction will be at any later time. During a measurement,
the change of the wavefunction into another one is not deterministic, but rather
unpredictable, i.e., random.
The probabilistic nature of
quantum mechanics thus stems from the act of measurement. This is one of the
most difficult aspects of quantum systems to understand. It was the central
topic in the famous Bohr-Einstein debates, in which the two
scientists attempted to clarify these fundamental principles by way of thought
experiments. In the decades after the formulation of quantum mechanics, the
question of what constitutes a "measurement" has been extensively studied. Interpretations of quantum
mechanics have been formulated to do away with the concept of "wavefunction
collapse"; see, for example, the relative state interpretation.
The basic idea is that when a quantum system interacts with a measuring
apparatus, their respective wavefunctions become entangled, so that the original
quantum system ceases to exist as an independent entity. For details, see the
article on measurement in quantum
mechanics.
In the mathematically rigorous formulation of quantum mechanics, developed by
Paul Dirac[7] and John von Neumann[8], the possible states
of a quantum mechanical system are represented by unit vectors (called "state
vectors") residing in a complex separable Hilbert space (variously called the "state space"
or the "associated Hilbert space" of the system) well defined up to a complex
number of norm 1 (the phase factor). In other words, the possible states are
points in the projectivization of a Hilbert space, usually
called the complex projective space. The exact
nature of this Hilbert space is dependent on the system; for example, the state
space for position and momentum states is the space of square-integrable
functions, while the state space for the spin of a single proton is just the
product of two complex planes. Each observable is represented by a maximally-Hermitian (precisely: by a self-adjoint) linear operator acting on the state space. Each eigenstate of
an observable corresponds to an eigenvector of the operator, and the associated eigenvalue
corresponds to the value of the observable in that eigenstate. If the operator's
spectrum is discrete, the observable can only attain those discrete
eigenvalues.
The time evolution of a quantum state is described by the Schrödinger
equation, in which the Hamiltonian, the operator
corresponding to the total energy of the system, generates time evolution.
The inner product between two state vectors is a
complex number known as a probability amplitude. During a
measurement, the probability that a system collapses from a given initial state
to a particular eigenstate is given by the square of the absolute value of the
probability amplitudes between the initial and final states. The possible
results of a measurement are the eigenvalues of the operator - which explains
the choice of Hermitian operators, for which all the eigenvalues are
real. We can find the probability distribution of an observable in a given state
by computing the spectral decomposition of the corresponding
operator. Heisenberg's uncertainty principle is represented by
the statement that the operators corresponding to certain observables do not commute.
The Schrödinger equation acts on the entire probability amplitude, not merely
its absolute value. Whereas the absolute value of the probability amplitude
encodes information about probabilities, its phase encodes information about the interference between quantum
states. This gives rise to the wave-like behavior of quantum states.
It turns out that analytic solutions of Schrödinger's equation are only
available for a
small number of model Hamiltonians, of which the quantum harmonic oscillator, the particle in a box,
the hydrogen molecular ion and the hydrogen atom are the most
important representatives. Even the helium atom, which contains just one more electron than
hydrogen, defies all attempts at a fully analytic treatment. There exist several
techniques for generating approximate solutions. For instance, in the method
known as perturbation theory one
uses the analytic results for a simple quantum mechanical model to generate
results for a more complicated model related to the simple model by, for
example, the addition of a weak potential energy. Another method is the
"semi-classical equation of motion" approach, which applies to systems for which
quantum mechanics produces weak deviations from classical behavior. The
deviations can be calculated based on the classical motion. This approach is
important for the field of quantum chaos.
An alternative formulation of quantum mechanics is Feynman's path integral formulation, in which a
quantum-mechanical amplitude is considered as a sum over histories between
initial and final states; this is the quantum-mechanical counterpart of action
principles in classical mechanics.
The fundamental rules of quantum mechanics are very broad. They assert that
the state space of a system is a Hilbert space and the observables are Hermitian operators acting on that space,
but do not tell us which Hilbert space or which operators, or if it even exists.
These must be chosen appropriately in order to obtain a quantitative description
of a quantum system. An important guide for making these choices is the correspondence principle, which states
that the predictions of quantum mechanics reduce to those of classical physics
when a system moves to higher energies or equivalently, larger quantum numbers.
In other words, classic mechanics is simply a quantum mechanics of large
systems. This "high energy" limit is known as the classical or
correspondence limit. One can therefore start from an established
classical model of a particular system, and attempt to guess the underlying
quantum model that gives rise to the classical model in the correspondence
limit.
When quantum mechanics was originally formulated, it was applied to models
whose correspondence limit was non-relativistic classical
mechanics. For instance, the well-known model of the quantum harmonic oscillator uses an
explicitly non-relativistic expression for the kinetic energy of the oscillator, and is thus a
quantum version of the classical harmonic oscillator.
Early attempts to merge quantum mechanics with special relativity involved the replacement
of the Schrödinger equation with a covariant equation such as the Klein-Gordon equation or the Dirac equation. While
these theories were successful in explaining many experimental results, they had
certain unsatisfactory qualities stemming from their neglect of the relativistic
creation and annihilation of particles. A fully relativistic quantum theory
required the development of quantum field theory, which applies
quantization to a field rather than a fixed set of particles. The first complete
quantum field theory, quantum electrodynamics, provides a
fully quantum description of the electromagnetic interaction.
The full apparatus of quantum field theory is often unnecessary for
describing electrodynamic systems. A simpler approach, one employed since the
inception of quantum mechanics, is to treat charged particles as quantum mechanical objects
being acted on by a classical electromagnetic field. For example, the elementary
quantum model of the hydrogen atom describes the electric field of the
hydrogen atom using a classical 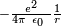 Coulomb potential. This "semi-classical" approach fails if quantum fluctuations
in the electromagnetic field play an important role, such as in the emission of
photons by charged particles.
Coulomb potential. This "semi-classical" approach fails if quantum fluctuations
in the electromagnetic field play an important role, such as in the emission of
photons by charged particles.
Quantum field theories for the strong nuclear
force and the weak nuclear force have been developed. The
quantum field theory of the strong nuclear force is called quantum
chromodynamics, and describes the interactions of the subnuclear particles:
quarks and gluons. The weak nuclear
force and the electromagnetic force were unified, in their quantized forms,
into a single quantum field theory known as electroweak theory,
by the physicists Abdus
Salam, Sheldon Glashow and Steven Weinberg.
It has proven difficult to construct quantum models of gravity, the remaining fundamental force. Semi-classical
approximations are workable, and have led to predictions such as Hawking radiation.
However, the formulation of a complete theory of quantum gravity is hindered by apparent
incompatibilities between general relativity, the most accurate theory
of gravity currently known, and some of the fundamental assumptions of quantum
theory. The resolution of these incompatibilities is an area of active research,
and theories such as string
theory are among the possible candidates for a future theory of quantum
gravity restricts the acceptable solutions of
the wavefunction. At each wall:
|
![]() Copyright(C) 2007
- 2015. All rights reserved.
Copyright(C) 2007
- 2015. All rights reserved.